top of page
Search


Nutraceutical Playbook Conclusion: Walter Healthcare- Accelerating India’s Global Nutraceutical Growth
The blog is the final segment of our nutraceutical playbook, highlighting brand building in Nutraceuticals, role of CDMOs in brand success, and why choose Walter as your partner.
Akshay Gautam
3 days ago9 min read


Nutraceutical Playbook Part 5: Walter Healthcare Building Market-Ready Nutraceutical Brands
The nutraceutical landscape has moved past simple growth and entered a critical "sophistication phase." Today's consumer is a powerful, highly informed individual, a meticulous label-reader who scrutinizes every claim and compares products with unprecedented diligence. This shift, coupled with stricter global regulatory oversight, has redefined the market. In this hyper-competitive new reality, the fundamental requirements are non-negotiable: strong, science-backed formulatio
Akshay Gautam
Feb 1410 min read


Nutraceutical Playbook Part 3: India’s Nutraceutical Regulations: Safety, Science, and Market Integrity
India’s healthcare narrative is undergoing a seismic, irreversible shift. The traditional, crisis-driven model is giving way to a new focus: proactive wellness, foundational nutrition, and holistic lifestyle management. This evolution is fueled by a confluence of factors, including rising health literacy, ubiquitous digital information, the surge in lifestyle disorders, and the sharpened post-pandemic awareness that health is an asset, not an afterthought.
Akshay Gautam
Jan 3110 min read


Nutraceutical Playbook Part 2: From Treatment to Prevention: Why Nutraceuticals Are Shaping the Future of Healthcare
This blog is the highlight of the changing healthcare landscape
from the clinical system to the preventive one. A cornerstone of this modern movement is the ascending role of nutraceuticals. These are not just simple supplements; they are scientifically engineered nutritional products specifically designed to bolster overall health, enhance the body's natural resilience, and maintain internal balance before the onset of disease. Consequently, nutraceuticals are evolving fro
Akshay Gautam
Jan 178 min read

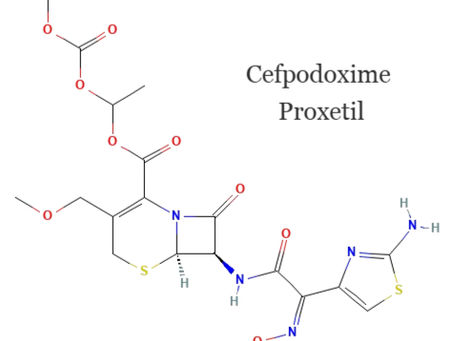
Cefpodoxime Proxetil Oral Suspension: Stability, Palatability, and Performance Redefined
This blog develops an insight about theCefpodoxime Proxetil is a prime example, standing out as a vital antibiotic in pediatrics due to its broad-spectrum efficacy, favorable safety profile, and simple dosing regimen. However, transforming the active pharmaceutical ingredient (API) into a stable, palatable, and patient-friendly oral suspension is a significant challenge in pharmaceutical engineering.
Akshay Gautam
Dec 2, 20256 min read


Amoxycillin-Clavulanic Acid Formulation: A Powerful Dual-Action Antibiotic Formula
It provides essential coverage for a wide range of conditions, from respiratory and skin infections to various mixed bacterial ailments across all age groups, particularly when single-agent antibiotics fail.
The power of this formulation lies in its strategic, dual-action mechanism. Amoxycillin offers potent, broad-spectrum bactericidal activity. Simultaneously, Clavulanic Acid acts as a guardian, breaking down the resistance mechanisms that bacteria use to neutralize the a
Akshay Gautam
Nov 26, 202511 min read


Understanding Evaporation in Pharmaceuticals
In the fast-evolving world of pharmaceutical manufacturing, the operation of evaporation is far more than simple solvent removal. It is a mission-critical, scientifically engineered transformation that directly determines product quality, therapeutic potency, and regulatory success. Achieving precise concentration through controlled evaporation is a cornerstone of product stability, bioavailability, shelf life, and successful downstream processing.
Akshay Gautam
Nov 15, 202511 min read


Beyond the Bubble: Effervescent Tablets in Pharmaceuticals
The blog is the quick idea for the basic idea about effervescent, what they are, how they work, basic insight for composition, and easy effective execution.
Akshay Gautam
Nov 8, 202510 min read


Dryers in Pharmaceutical Manufacturing: Ensuring Efficiency, Quality, and Product Stability
The blog is a quick insight into the process of the pharmaceutical drying. highlighting its importance alongside the types of the frequently used dryers, making the process effective and engaging with the development of a stable, quality product.
Akshay Gautam
Nov 1, 202512 min read


WHO GMP vs Indian GMP: A Manufacturer’s Guide to GMP
the blog provide idea about Good Manufacturing Practices (GMP) are the foundation of quality in the pharmaceutical industry. They ensure that every medicine is consistently safe, effective, and of high quality. This commitment to excellence protects public health and builds trust in medicines worldwide.
Globally, the World Health Organization (WHO) provides comprehensive GMP guidelines. The most important ones are TRS 986 Annex 2 for general GMP and TRS 1044 Annex 2 for ster
Akshay Gautam
Oct 18, 202513 min read


Healing Without Harming: Sustainable Pharma for a Greener Tomorrow
the blog provides idea about the pharmaceutica industry, while saving lives, also leaves a heavy ecological footprint through high water usage, energy demands, plastic packaging, chemical waste, and global supply chains. This blog explores how sustainability is transforming pharma, from green chemistry and renewable energy to eco-friendly packaging innovations like glass jars, aluminum caps, and paper-based blisters. It reviews regulatory frameworks in India and globally, whi
Akshay Gautam
Sep 6, 20257 min read


CDSCO & ICH Guidelines on Packaging for Stability Testing
Pharmaceutical packaging: not just a container, but a vital protector. It's the silent guardian ensuring your medicine remains safe,...
Akshay Gautam
Aug 30, 20258 min read


Mandatory CDSCO Guidelines For Labeling Compliance in India
Ensuring every pharmaceutical label meets CDSCO’s strict standards isn’t optional; it’s the key to patient safety, regulatory approval,...
Akshay Gautam
Aug 16, 20256 min read


CDSCO VS State FDA: Approval Authority in India
Navigate the distinct roles of CDSCO and State FDA to ensure faster approvals, full compliance, and successful market entry. 1....
Akshay Gautam
Aug 8, 202510 min read


GLP Series (Schedule L): 11 Internal Quality System Audits
In the intricate landscape of laboratory operations, maintaining the highest standards of quality is paramount. Internal quality system...
Marketing - Walter®
Feb 8, 20242 min read


GLP Series (Schedule L): 9 Microbiological Cultures
Microbial cultures are vital in scientific research, especially in pharmaceuticals and biotech. Effective management through maintenance,...
Ashima Thakur
Dec 2, 202313 min read


GMP Series (Schedule M): 6. Personnel
Ensuring the integrity and excellence of pharmaceutical manufacturing: A comprehensive guide to personnel criteria in maintaining...
Jyotsna Sharma
Nov 23, 202312 min read


GMP Series (Schedule M): 5. Quality Control area
Quality control is a critical aspect of any manufacturing or production process, ensuring that products meet the required standards and...
Jyotsna Sharma
Nov 18, 202312 min read


GLP Series (Schedule L): 7 Maintenance, Calibration, and Validation of Equipments
Equipment maintenance and calibration are top priorities to ensure precision and reliability in laboratory operations, demonstrating...
Ashima Thakur
Nov 18, 202312 min read


GMP Series (Schedule M): 4. Ancillary Areas
In pharmaceutical manufacturing, strict rules enforce the separation of ancillary areas from restrooms, maintenance zones, and animal...
Jyotsna Sharma
Nov 5, 202312 min read
Home >> Pharma News & Blog
bottom of page