Mandatory CDSCO Guidelines For Labeling Compliance in India
- Akshay Gautam
- Aug 16, 2025
- 6 min read
Ensuring every pharmaceutical label meets CDSCO’s strict standards isn’t optional; it’s the key to patient safety, regulatory approval, and brand trust in India’s competitive market.
1. Introduction
In the ever-changing pharmaceutical market, product labeling is more than just a marketing element; it is a legislative requirement that ensures patient safety, promotes product transparency, and facilitates the traceability of the product throughout the entire supply chain. In an increasingly competitive pharmaceutical landscape, the industry faces a growing challenge with counterfeit labeling and a lack of readily available, up-to-date information. To combat this, the Central Drugs Standard Control Organization (CDSCO) maintains rigorous oversight, enforcing strict drug labeling guidelines under the foundational Drugs and Cosmetics Act of 1940 and the Drugs and Cosmetics Rules of 1945 for all categories of drug products. It includes the important details in terms of active ingredient, dosage forms, manufacturer’s information, batch number, manufacturing, expiry dates, storage conditions, and statutory warnings.
The CDSCO’s standards are primarily outlined in Schedule 96 (minimum information required on labeling), followed by Schedules H and H1 (product sales with detailed prescriptions by a registered practitioner), and Schedule X (drugs that require strict control due to their potential for abuse). In case of any kind of failure in the execution of the labeling law, which can result in product recalls, penalties, or suspension of licenses.
At Walter Healthcare, we ensure complete labeling compliance for pharmaceutical companies, from regulatory interpretation to pre-market approval, helping them avoid errors and remain audit-ready for the long-term profitable execution of the product. The post will delve into the regulatory framework, mandatory elements, common pitfalls, dos and don'ts, and best practices, providing a practical guide to achieving and maintaining CDSCO labeling compliance in India.
2. Regulatory Basis for Labeling Compliance
2.1 Legal Framework
Before the Drugs Act of 1940, India's drug regulations were scarce, with existing laws like the Indian Merchandise Act and Opium Act only vaguely addressing opium and general product misbranding. The pharmaceutical labeling process in India has undergone significant improvement, largely due to two crucial pieces of legislation.
Drugs and Cosmetics Act 1940: The Act ensures product safety by mandating accurate, comprehensive, and clear labeling for both drugs and cosmetics in India, thereby ensuring the safety, efficacy, and quality of pharmaceutical products.
Drug and Cosmetics Rule 1945: In India, all drug and cosmetic products are subject to mandatory labeling and packaging regulations, as stipulated in Part 9 of the rules. These guidelines cover aspects such as import details, quantity, locations, labeling specifics, and expiry dates. The primary goal is to provide consumers with precise information, including the product's name, ingredients, manufacturer, batch information, expiry date, and clear usage instructions.
2.2 CDSCO’s Role
The Central Drugs Standard Control Organisation (CDSCO) is India's national pharmaceutical regulatory body. It operates under the Drugs and Cosmetics Act of 1940 and the Drugs and Cosmetics Rules of 1945, with specific legal provisions supporting its labeling-related functions.
At the time of the approval process of New Drugs (ND), Subsequent New Drugs (SND), and Fixed Dose Combination (FDC) authority is responsible for approval of labeling content.
Before providing market authorization verification of labeling content for imported products, generics, and biological products being done.
Rule 24 and 24A- Import license application requirements (including label compliance).
For preventing misleading or incomplete labeling, CDSCO conducts inspections and enforcement action.
Rule 85- Licensing authority’s power to suspend or cancel licenses for non-compliance.
Rule 150E- Provisions for recall and enforcement.
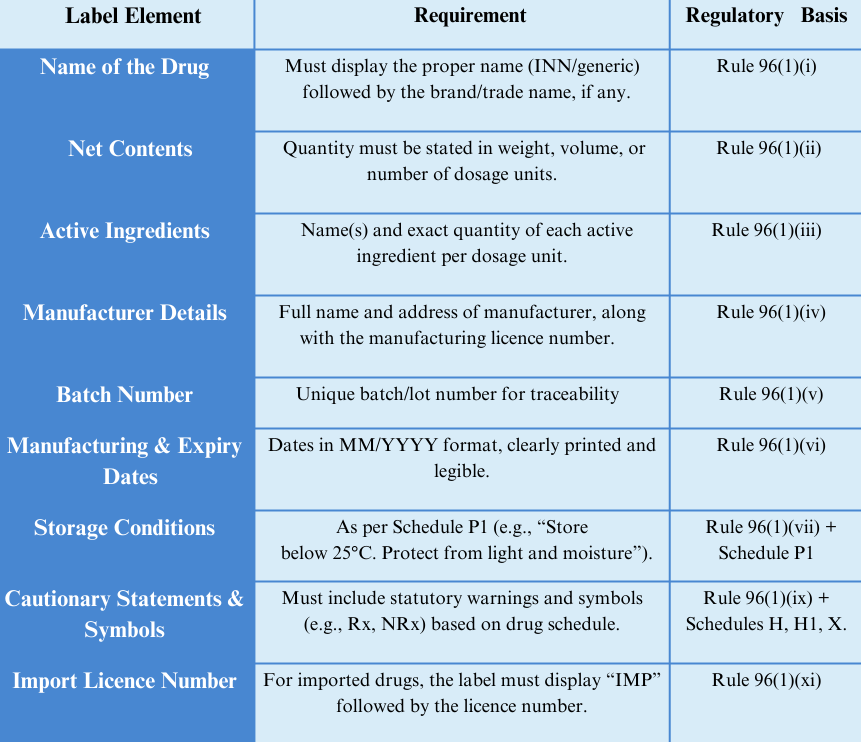
3. Key Mandatory Labeling Requirements under CDSCO
5. Common Non-Compliance Issues in Labeling
Despite CDSCO's clear guidelines, labeling errors remain the primary reason for regulatory actions within the Indian pharmaceutical sector. Violations, including absent statutory details or improperly formatted warnings, threaten compliance, prolong market approvals, and endanger patient safety. Gaining a comprehensive understanding of frequent errors is essential to avoid costly enforcement measures.
Missing Manufacturing License Number: Product traceability is affected due to the omission of the manufacturing license number, a requirement under Rule 96 (1)(iv).
Incorrect Expiry Date Format: Expiry dates are printed in formats other than the mandated MM/YYYY (e.g., DD/MM/YYYY), which can lead to confusion.
Improper or Missing Warning Statements: Statutory warnings for Schedule H, H1, and X are either missing, incomplete, altered, or abbreviated, violating Rule 96 (1)(ix) and respective schedules.
Unreadable or Small Font Sizes: Crucial information like dosage instructions, expiry dates, and warnings is printed in very small font sizes or with low contrast, making it difficult to read.
Misplaced or Incorrect Regulatory Symbols: “Rx” or “XRx” symbols are missing, wrongly positioned, printed in the wrong color, or incorrectly sized, breaching the specifications of Rule 96 (1)(ix) and drug schedules.
6. Do’s and Don'ts of Labeling

7. Best Practices for Label Compliance
Implement a comprehensive CDSCO labeling checklist for every batch: Develop a detailed, product-specific checklist that includes all mandatory information, such as brand name, generic name, manufacturing license number, batch number, manufacturing date, expiry date, storage conditions, Rx/XRx symbols, and cautionary statements. Regularly update this checklist to align with the latest CDSCO requirements, using it as a quality control measure to guarantee accurate label printing.
Conduct internal audits before product release: Establish a multi-level review process where regulatory, quality, and production teams independently verify labels. This helps identify inconsistencies like font size issues, misplaced symbols, missing regulatory statements, or incorrect artwork before products leave the facility.
Stay continuously updated on CDSCO labeling changes: Monitor CDSCO's official website, Gazette notifications, and circulars to track any modifications in labeling requirements. Designate a team member to disseminate these updates internally, ensuring all stakeholders, including designers, printers, and quality assurance personnel, are aligned with the most recent rules and regulations.
Regularly engage with experienced consultants for compliance reviews: Utilize the expertise of external regulatory specialists to conduct periodic labeling compliance audits. Their experience with various clients and evolving CDSCO practices can help uncover blind spots, prepare for inspections, and prevent costly product recalls or regulatory actions.
8. Why Walter Healthcare for labeling compliance
Compliance from the Ground Up: At Walter Healthcare, we prioritize regulatory compliance from the initial stages of pharmaceutical manufacturing. Our approach ensures that all labeling, documentation, and design adhere to CDSCO norms right from formulation development.
Proactive Regulatory Expertise: Our in-house regulatory team diligently monitors all CDSCO circulars, notifications, and bans. This vigilance allows us to proactively update labeling guidelines, guaranteeing continuous compliance even as regulations evolve.
Global Quality Standards and Certifications: Walter's manufacturing plants hold prestigious certifications, including cGMP, WHO-GMP, and FDA-GLP. This robust infrastructure underscores our strict adherence to international quality and regulatory standards, supporting accurate and compliant labeling across all batches.
Comprehensive Audit-Ready Operations: Through both internal and external audits, Walter Regulatory ensures that all regulatory obligations, including CDSCO labeling requirements, are consistently met.
End-to-End Labeling Solutions: We provide comprehensive packaging and batching services, encompassing labeling for all dosage forms such as vials, injectables, sachets, and blister packs. This guarantees consistent labeling compliance across diverse formats.
Your Strategic, One-Stop Contract Pharma Partner: As a leading CDMO, Walter offers integrated manufacturing solutions under one roof. From formulation development and packaging to labeling, regulatory documentation, and dispatch, we streamline the labeling compliance process while maintaining stringent control over quality and regulatory requirements.

9. Conclusion
In India's rapidly evolving pharmaceutical landscape, labeling compliance is paramount for patient safety, market credibility, and business continuity. The CDSCO, through the stringent rules of the Drugs and Cosmetics Act of 1940 and Rules of 1945, mandates that every drug label provide complete, accurate, and easily understandable information to prevent misinterpretation or misuse. Non-compliance can lead to costly recalls, legal penalties, and reputational damage.
Walter Healthcare serves as a strategic partner in this critical area. We integrate compliance from the initial stages of product development and maintain proactive regulatory surveillance. Adhering to global quality standards such as cGMP, WHO-GMP, and FDA-GLP, we ensure every label is audit-ready and fully aligned with CDSCO norms. With our comprehensive labeling solutions, robust audits, and expertise in adapting to evolving laws, Walter Healthcare empowers pharmaceutical brands to not only meet regulatory requirements but also anticipate and mitigate risks. We are more than just a manufacturer; we are your trusted partner in safeguarding your products and reputation within the global marketplace.









Comments