CDSCO & ICH Guidelines on Packaging for Stability Testing
- Akshay Gautam
- Aug 30, 2025
- 8 min read
Pharmaceutical packaging: not just a container, but a vital protector. It's the silent guardian ensuring your medicine remains safe, potent, and effective, safeguarding its integrity from production to your hands.
1. Introduction
In the pharmaceutical industry, product stability is paramount. It’s not merely a regulatory checkbox; it’s a steadfast promise of a product’s effectiveness, dependability, and safety throughout its entire shelf life. Packaging, particularly with a meticulously designed closure system, acts as an indispensable guardian, preserving potency by shielding against the relentless assaults of oxygen, moisture, light, and extreme temperatures. Without adequate packaging, drug quality can deteriorate long before the expiration date, thereby undermining its efficacy.
The intricate landscape of packaging assessment in stability testing is primarily shaped by two influential regulatory pillars: the International Council for Harmonisation (ICH) guidelines and the Central Drugs Standard Control Organisation (CDSCO) rules. Both authorities underscore the critical importance of packaging, though their approaches diverge in scope, climate considerations, and strategic execution. ICH guidelines offer a global perspective, while CDSCO rules are acutely focused on the nuances of the Indian pharmaceutical market.
For pharmaceutical organizations, adherence to these rigorous requirements transcends mere obligation; it's a strategic imperative. Compliance accelerates approvals, mitigates regulatory risks, and fortifies a global competitive edge. Walter Healthcare stands as a crucial partner in this intricate process, seamlessly integrating deep regulatory insight with cutting-edge scientific packaging solutions. This empowers pharmaceutical innovators to forge packaging strategies that not only satisfy ICH's international benchmarks but also meet CDSCO’s specific mandates, enabling companies to confidently propel their products from the development phase to a successful market launch.
This blog post will delve into the pivotal roles of ICH and CDSCO in packaging for stability testing, highlighting their intricate similarities and distinct differences. We will explore why execution is paramount and how Walter Healthcare skillfully bridges the gap between global harmonization and local compliance.
2. The Role of Packaging in Stability Testing
Packaging is far more than a container; it's a critical factor that directly influences the safety, efficacy, and shelf life of pharmaceutical products. In stability testing, the packaging plays a pivotal role in determining how a drug product holds up under various environmental conditions over time. As per both CDSCO (Central Drugs Standard Control Organization) and ICH (International Council for Harmonisation) guidelines, evaluating the interaction between a drug and its packaging is not just a good practice regulatory requirement.
2.1 Why Packaging Matters in Stability Studies
The primary function of pharmaceutical packaging is to protect the product from physical, chemical, and biological degradation. During stability testing, the selected packaging must simulate real-world storage and distribution conditions. The right packaging ensures that:
Moisture, oxygen, and light do not compromise product stability.
There is no leaching or migration of packaging components into the drug product.
The drug does not adsorb onto the container walls or chemically react with the packaging material.
The product retains its identity, strength, quality, and purity throughout its shelf life.
2.2 Types of Packaging in Stability Testing
Depending on the dosage form, packaging can include:
Blister packs for tablets and capsules
Glass or plastic vials for injectables
Bottles, tubes, sachets, or ampoules for liquids, creams, and powders
Each packaging type comes with its own set of challenges and considerations. For instance, plastic containers may allow moisture ingress, while glass containers could leach alkali substances if not treated correctly. That’s why selecting the right material, design, and closure system is crucial in stability testing.
2.3 Packaging as a Part of Product Development
In modern pharmaceutical development, packaging is no longer an afterthought. It is integrated early in the development cycle and tested under accelerated, intermediate, and long-term stability conditions. This proactive approach helps mitigate risks and avoid costly recalls or shelf-life reductions post-approval.
2.4 Regulatory Expectations: CDSCO and ICH
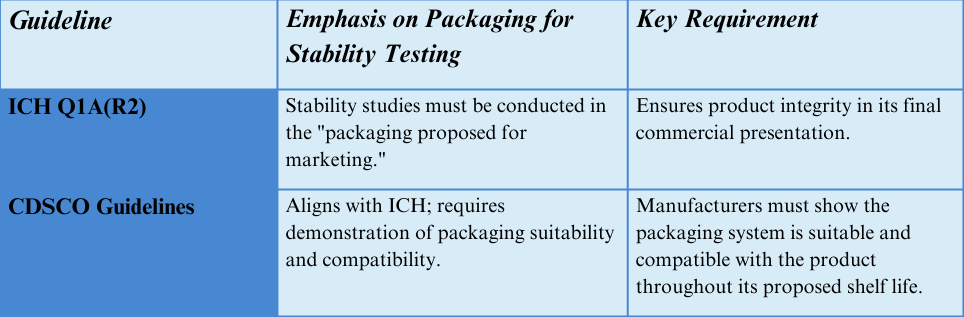
Both CDSCO and ICH guidelines highlight the need to conduct stability studies using the final packaging configuration intended for marketing. This means the container-closure system used in stability testing must be identical to what will be used commercially. Here’s what the guidelines emphasize:
ICH Q1A(R2) states that the stability studies should be conducted in the “packaging proposed for marketing” to ensure product integrity.
The CDSCO Guidelines also align with this approach, requiring manufacturers to demonstrate that the packaging system is suitable and compatible with the product over its proposed shelf life.
3. ICH Guidelines for Packaging in Stability Testing
The International Council for Harmonisation (ICH) plays a central role in harmonizing regulatory requirements across major global markets. Among its core areas of focus is ensuring that pharmaceutical products remain safe, effective, and high-quality throughout their shelf life, something that cannot be achieved without robust packaging systems.
In stability testing, the ICH guidelines offer a clear, science-driven framework for evaluating how packaging influences the physical, chemical, biological, and microbiological integrity of drug products over time.
3.1 Key ICH Guidelines Relevant to Packaging
The ICH provides several guidance documents that touch upon packaging within the context of stability testing. Let’s explore the most relevant ones:
ICH Q1A(R2) - Stability Testing of New Drug Substances and Products
This is the cornerstone guideline for stability testing. It outlines general principles and protocols, including the requirement that stability testing be conducted in the packaging proposed for marketing. The key expectations include:
Using the final container-closure system during stability studies to simulate actual storage conditions.
Justifying the choice of packaging materials based on protective properties such as resistance to moisture, oxygen, light, or mechanical stress.
Including accelerated and long-term data in the intended packaging to establish a reliable shelf life.
ICH Q1B - Photostability Testing
Photostability testing evaluates the effect of light exposure on drug products. Here, packaging is critical:
The product must be tested in its market-intended packaging, especially if the formulation is photosensitive.
The container should offer adequate light protection (e.g., amber glass or opaque blister) if degradation is observed during light exposure.
ICH Q5C - Stability Testing of Biotechnological/Biological Products
For biologics, packaging requirements are even more stringent due to the sensitive nature of proteins, peptides, and other complex molecules. ICH Q5C emphasizes:
The need for packaging systems that ensure sterility, compatibility, and stability.
Assessment of interaction between the product and the container, such as adsorption to vial surfaces or interaction with rubber stoppers.
3.2 Packaging Integrity: A Non-Negotiable Requirement
According to ICH guidance, the container-closure system must provide consistent protection against environmental conditions over time. This includes:
Barrier properties (moisture, oxygen, light)
Mechanical integrity (leak-proof seals, proper closures)
Chemical compatibility (no leachables, extractables, or degradation)
Microbiological safety (particularly for sterile and biologic products)
ICH also encourages manufacturers to perform container-closure integrity testing (CCIT) to ensure there’s no breach in packaging during the product’s shelf life.
3.3 Why ICH’s Packaging Guidance Matters
Global regulators, including the US FDA, EMA, PMDA, and CDSCO, follow ICH guidelines as the gold standard for product registration. Failure to adhere to packaging requirements in stability studies can lead to:
By incorporating ICH guidance from early development stages, pharmaceutical companies can mitigate risk, support global submissions, and deliver products that remain safe, effective, and reliable until the very end of their shelf life.
4. CDSCO Guidelines for Packaging in Stability Testing
When it comes to ensuring drug safety and efficacy in India, the Central Drugs Standard Control Organization (CDSCO) serves as the national regulatory authority, and its guidelines on packaging and stability testing are a crucial part of product approval. While the CDSCO aligns closely with international standards, such as the ICH, it also outlines country-specific expectations that Indian pharmaceutical manufacturers must meet to ensure their packaging systems are up to standard for stability evaluation.
4.1 Why Packaging is Central to CDSCO Stability Requirements
CDSCO recognizes that packaging is more than just a physical enclosure; it's a functional component of the drug product. Packaging must preserve the product's quality and integrity under Indian climatic conditions, particularly Zone IVb, characterized by high temperature and high humidity. As such, CDSCO mandates that stability testing be conducted using the actual packaging configuration intended for marketing in India. This ensures that the drug will remain stable throughout its shelf life in real-world storage and distribution environments.
4.2 Key CDSCO Expectations on Packaging in Stability Studies
Here’s what CDSCO emphasizes when it comes to packaging and stability:
Use of Market-Intended Packaging
Stability studies must be conducted using the same container-closure system proposed for commercial distribution.
Any changes in packaging (e.g., material type, closure system, blister type) require new or supporting stability data.
Climatic Zone IVb Conditions
India falls under Zone IVb (30°C ± 2°C / 75% RH ± 5% RH), meaning packaging must offer robust protection against heat and moisture.
CDSCO expects stability testing data under these conditions for both accelerated and long-term studies.
Packaging Material Selection
The choice of packaging must consider:
Barrier properties (against light, oxygen, and moisture)
Chemical compatibility with the formulation
Physical integrity during transportation and handling
Packaging components like closures, liners, blister foils, bottles, and ampoules must be evaluated for potential leachables, extractables, and interaction with the drug product.
Labelling and Tamper Evidence
Packaging used during stability studies must include complete labeling and should reflect the final market presentation, including tamper-evident features, where applicable.
4.3 CDSCO vs ICH: Alignment with Local Relevance
While CDSCO mirrors ICH principles in many respects, especially ICH Q1A(R2), its key distinction lies in adaptation to local climate and regulatory priorities:

4.4 Regulatory Consequences of Poor Packaging Practices
Inadequate or non-compliant packaging during stability testing can result in:
That’s why CDSCO urges pharmaceutical companies to consider packaging as early as the formulation development stage, not just as a post-formulation afterthought.
Packaging is a Regulatory Priority
In India, CDSCO requires that your product is not only pharmaceutically sound but also that its packaging actively preserves that quality over time. Adhering to CDSCO’s packaging guidelines during stability testing ensures compliance, fosters trust with regulators and healthcare providers, and ultimately safeguards patient health.
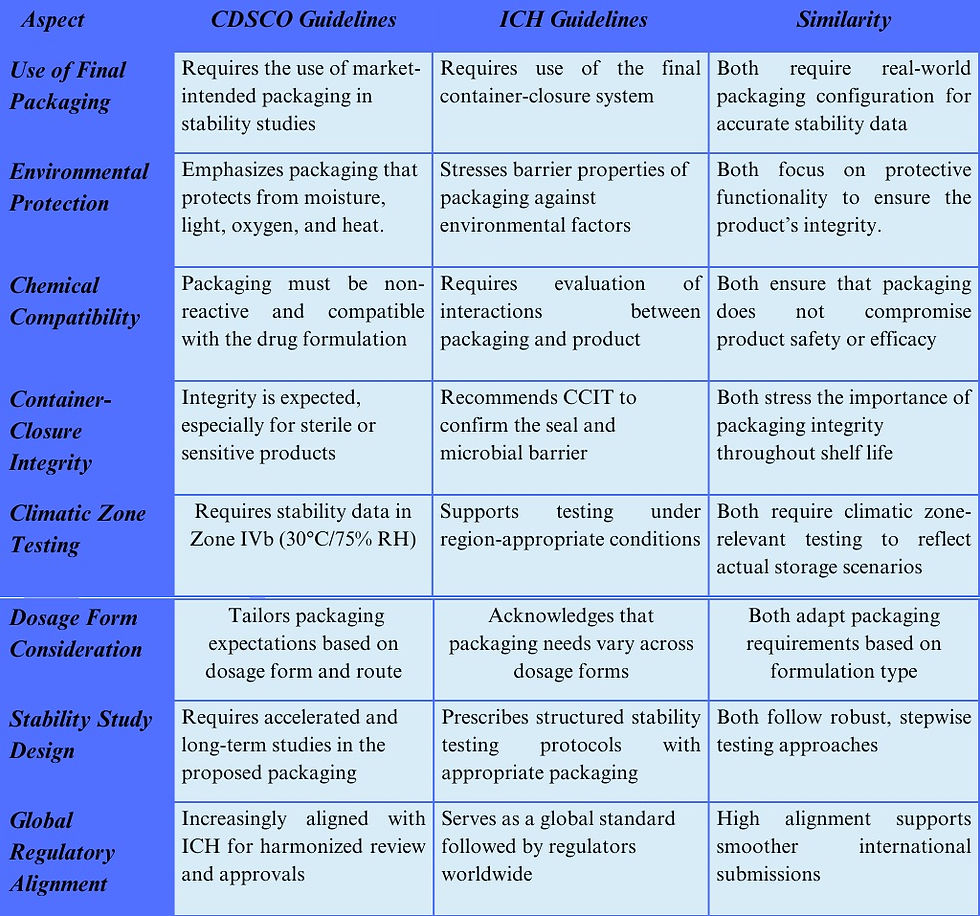
5. Similarities Between CDSCO and ICH Guidelines on Packaging in Stability Testing
While CDSCO (India’s Central Drugs Standard Control Organization) and the ICH (International Council for Harmonisation) largely align on the principles of pharmaceutical packaging in stability testing, there are distinct regional differences that companies must navigate to ensure regulatory compliance across markets.
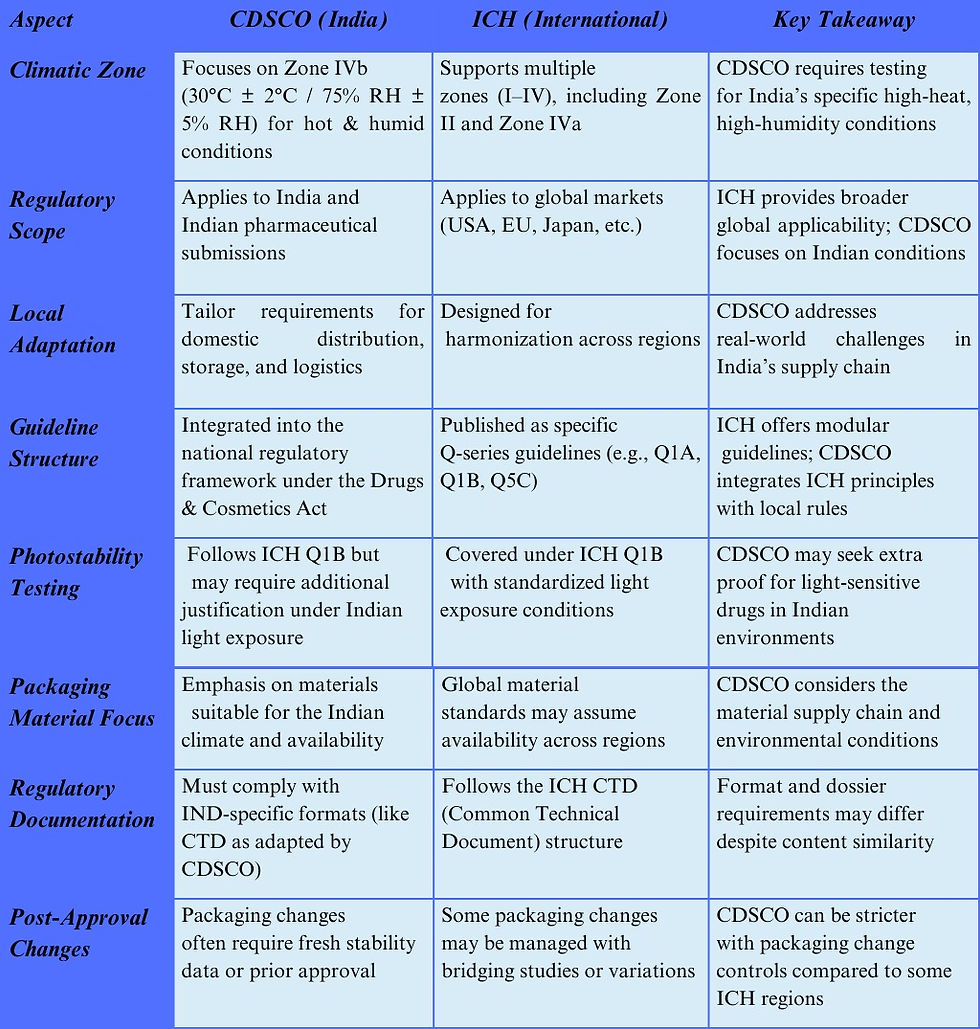
6. Differences Between CDSCO and ICH Guidelines on Packaging in Stability Testing
While CDSCO (India’s Central Drugs Standard Control Organization) and the ICH (International Council for Harmonisation) largely align on the principles of pharmaceutical packaging in stability testing, there are distinct regional differences that companies must navigate to ensure regulatory compliance across markets.
7. How Walter Healthcare Navigates CDSCO and ICH Guidelines
As a company committed to international quality standards and deep-rooted in the Indian healthcare ecosystem, Walter Healthcare develops its packaging strategies by aligning with ICH’s harmonized global framework while also addressing CDSCO’s India-specific requirements.
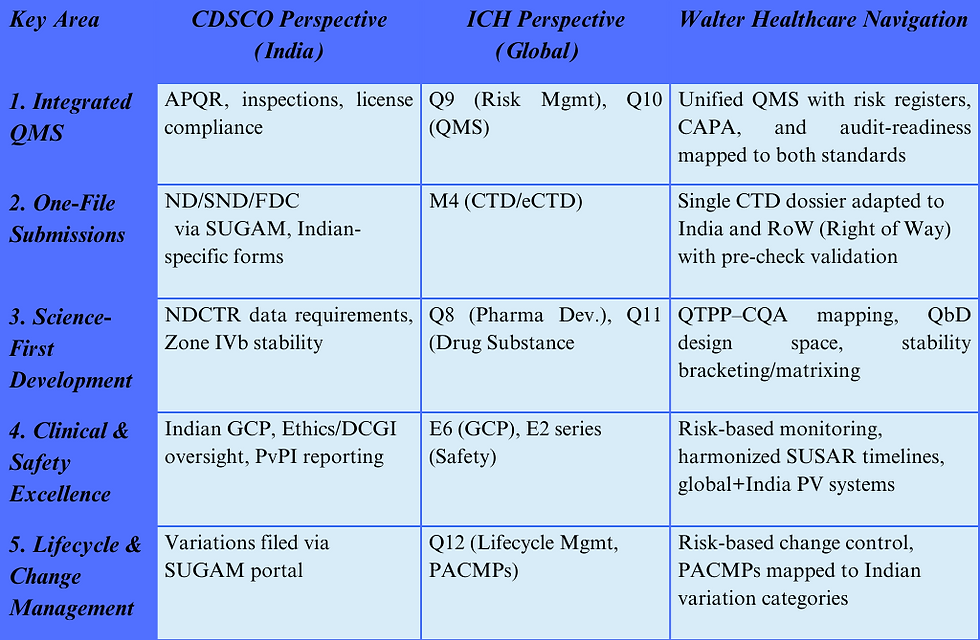
7.1 Walter Healthcare: Bridging Global and Local Standards
At Walter Healthcare, our quality-first philosophy extends to meticulous regulatory compliance. We guarantee that all products, whether for the Indian market or global submission, proactively meet the regulatory expectations of both CDSCO and ICH. This commitment ensures:
Robust Stability Data: Our data is region-appropriate and prepared for regulatory scrutiny.
Resilient Packaging: Designed to withstand real-world conditions.
By harmonizing global ICH guidelines with local CDSCO requirements, Walter Healthcare delivers pharmaceutical products that are compliant, stable, and trusted worldwide.
8. Conclusion
Packaging is not merely a vessel; it is a silent guardian of a drug’s quality, safety, and efficacy throughout its shelf life. Both ICH and CDSCO recognize this critical role, but while ICH provides a globally harmonized framework, CDSCO brings the local dimension of India’s climatic realities and regulatory expectations. Together, they ensure that medicines remain effective from manufacturing to the patient’s hands. At Walter Healthcare, we embrace this dual perspective. By embedding ICH best practices into our global dossier strategy and integrating CDSCO’s Zone IVb-specific requirements, we design packaging solutions that are not only regulator-approved but also patient-trusted. This balanced approach reduces approval risks, accelerates time-to-market, and strengthens confidence in product reliability. In an increasingly competitive pharmaceutical landscape, harmonizing international and local packaging standards is no longer optional; it is a strategic necessity. Walter Healthcare stands at this intersection, enabling innovators to deliver therapies that remain safe, stable, and effective across geographies.




Comments